Recently, Icatibant Injection developed by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. has been approved by the U.S. Food and Drug Administration (FDA).
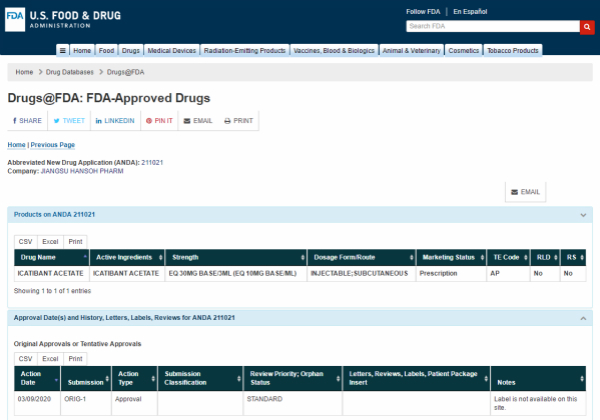
The drug is used to treat acute onset of hereditary angioedema (HAE) in adults by inhibiting the binding of bradykinin to B2 receptor to treat clinical symptoms of acute onset of HAE.
